| Kidney Res Clin Pract > Volume 40(3); 2021 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
The research was funded by Hallym University Research Fund (HURF-2018-29), the Korean Association of Internal Medicine (2018-180), the Korean Society of Nephrology, and National Research foundation of Korea (2016R1C1B1015687).
Authors’ contributions
Conceptualization: SK, SHB
Investigation: All authors
Data curation, Formal analysis: SG, SK, SHB
Administrative, Technical, or Material support: HES, JYR, HY, SRC, JWS, YHJ, JRK
Funding acquisition: SHB
Writing–original draft: SG, SK, SHB
Writing–review & editing: All authors
Figure 1.
Study population algorithm.

Figure 2.
Box plot for copeptin levels (A) and copeptin-to-urine Na × 100 (B) according to etiologies of hyponatremia.

Figure 3.
Area under receiver operating characteristics curve for insufficient effective arterial blood volume (secondary copeptin release).

Figure 4.
Outcomes within 6 hours (A) and 24 hours (B) stratified by copeptin level at baseline (below/above median).
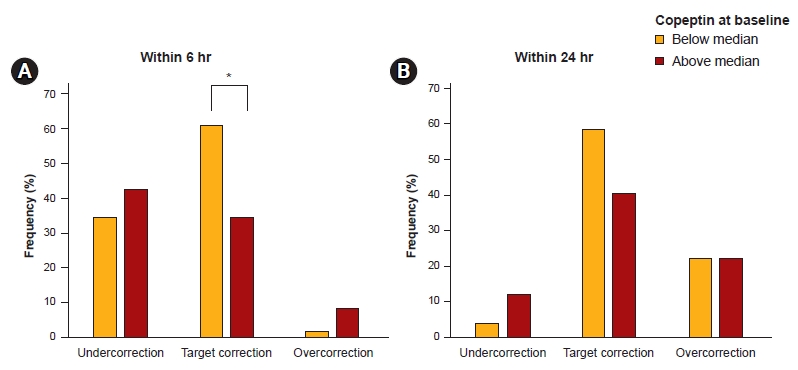
Figure 5.
Outcomes within 24 to 48 hours stratified by copeptin level at 24 hours after treatment (below/above median).

Table 1.
Table 2.
Table 3.
Table 4.
| Parameter | OR (95% CI) | p-value |
|---|---|---|
| Univariable analysis | ||
| Age | 1.00 (0.97–1.04) | 0.88 |
| Male sex | 1.00 (0.45–2.21) | >0.99 |
| Hypertension | 0.84 (0.36–1.92) | 0.67 |
| Diabetics vs. non-diabetics | 1.50 (0.62–3.62) | 0.37 |
| Cancer | 0.89 (0.35–2.27) | 0.81 |
| Cause of hyponatremia | 0.76 | |
| Body mass index | 1.02 (0.94–1.11) | 0.68 |
| Systolic blood pressure | 0.99 (0.97–1.01) | 0.27 |
| Serum Na | 0.85 (0.78–0.94) | 0.001* |
| Serum K | 0.49 (0.26–0.95) | 0.03* |
| Serum osmolality | 1.02 (1.00–1.05) | 0.064 |
| Serum creatinine | 1.97 (1.00–3.86) | 0.05 |
| Serum uric acid | 1.20 (1.00–1.44) | 0.05 |
| Serum C-reactive protein | 1.00 (0.99–1.02) | 0.64 |
| Urine osmolality | 1.00 (1.00–1.00) | 0.74 |
| Urine Na | 0.99 (0.99–1.00) | 0.16 |
| Urine K | 1.04 (1.01–1.06) | 0.002* |
| Multivariable analysis | ||
| Serum Na | 0.82 (0.73–0.93) | 0.002* |
| Serum creatinine | 2.91 (1.22–6.95) | 0.016* |
| Urine K | 1.05 (1.02–1.09) | 0.002* |
Table 5.
| Variable | Number (low, high) |
Univariable |
Multivariablea |
Multivariableb |
|||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Copeptin at baseline | |||||||
| Under correction within 6 hr | (17/49, 21/49) | 0.71 (0.31–1.60) | 0.41 | 0.70 (0.31–1.60) | 0.40 | 0.32 (0.10–1.02) | 0.05 |
| Target correction within 6 hr | (30/49, 17/49) | 2.97 (1.31–6.76) | 0.009* | 3.12 (1.35–7.22) | 0.008* | 2.97 (1.16–7.64) | 0.02* |
| Overcorrection within 6 hr | (1/49, 4/49) | 0.23 (0.03–2.18) | 0.20 | 2.23 (0.02–2.24) | 0.20 | 0.00 (0.00) | >0.99 |
| Under correction within 24 hr | (2/49, 6/49) | 0.31 (0.06–1.59) | 0.16 | 0.31 (0.06–1.64) | 0.17 | 0.00 (0.00) | 0.99 |
| Target correction within 24 hr | (26/49, 18/49) | 1.95 (0.87–4.37) | 0.11 | 2.01 (0.88–4.57) | 0.10 | 6.21 (1.67–23.09) | 0.006* |
| Overcorrection within 24 hr | (11/49, 11/49) | 1.00 (0.39–2.58) | >0.99 | 0.92 (0.34–2.51) | 0.87 | 0.44 (0.07–2.77) | 0.379 |
| Copeptin at 24 hr after treatment | |||||||
| Under correction within 24–48 hr | (9/47, 19/50) | 0.39 (0.15–0.97) | 0.04* | 0.38 (0.15–0.97) | 0.04* | 0.28 (0.03–2.49) | 0.25 |
| Target correction within 24–48 hr | (27/47, 27/50) | 1.15 (0.52–2.57) | 0.733 | 1.16 (0.52–2.59) | 0.72 | 0.77 (0.25–2.33) | 0.64 |
| Overcorrection within 24–48 hr | (10/47, 2/50) | 6.49 (1.34–31.42) | 0.02p | 6.49 (1.34–31.42) | 0.02* | 18.00 (1.59–203.45) | 0.02* |
b analyzed with age, sex, body mass index, systolic blood pressure, diabetes mellitus, liver cirrhosis, cancer, serum sodium/creatinine/uric acid/C-reactive protein/serum osmolality/urine osmolality/urine sodium at baseline, cause of hyponatremia, hypertonic saline volume, and infusion mode of hypertonic saline (bolus/continuous/mixed).
References
- TOOLS
-
METRICS

- ORCID iDs
-
Suryeong Go

https://orcid.org/0000-0001-8118-7994Sejoong Kim

https://orcid.org/0000-0002-7238-9962Hyung-Eun Son

https://orcid.org/0000-0002-8719-3823Ji-Young Ryu

https://orcid.org/0000-0003-4134-1007Huijin Yang

https://orcid.org/0000-0001-5885-1519Sun Ryoung Choi

https://orcid.org/0000-0002-9668-3349Jang-Won Seo

https://orcid.org/0000-0002-3495-5388You Hwan Jo

https://orcid.org/0000-0002-9507-7603Ja-Ryong Koo

https://orcid.org/0000-0003-4245-2569Seon Ha Baek

https://orcid.org/0000-0002-4751-9817 - Related articles
-
Association between hearing loss and physical performance in patients on maintenance hemodialysis



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















