| Kidney Res Clin Pract > Volume 39(3); 2020 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
This research was supported by a grant (NRF-2019R1G1 A1099728) from the National Research Foundation (NRF) of Korea and a grant (No. 2019M3E5D1A02069071) from the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT). This study was also supported by the Soonchunhyang University Research Fund.
Authors’ contributions
Ji-Hye Lee and Si-Hyong Jang reviewed biopsies. Nam-Jun Cho, Nam Hun Heo, and Samel Park collected data and performed statistical analysis. Ji-Hye Lee and Samel Park conceptualized this study and drafted the manuscript. Hyo-Wook Gil, Eun Young Lee, and Jong-Seok Moon advised on the study.
Figure 1
Representative histology by light microscopy of glomeruli with (A) mesangial hypercellularity and (B) endocapillary hypercellularity.

Figure 2
Representative images of electron microscopy of (A) rare, (B) mild, (C) moderate, and (D) diffuse foot process effacement.
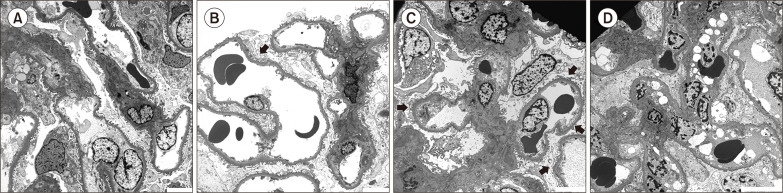
Figure 3
Characteristics of renal histology according to stratification of severity of foot process effacement.
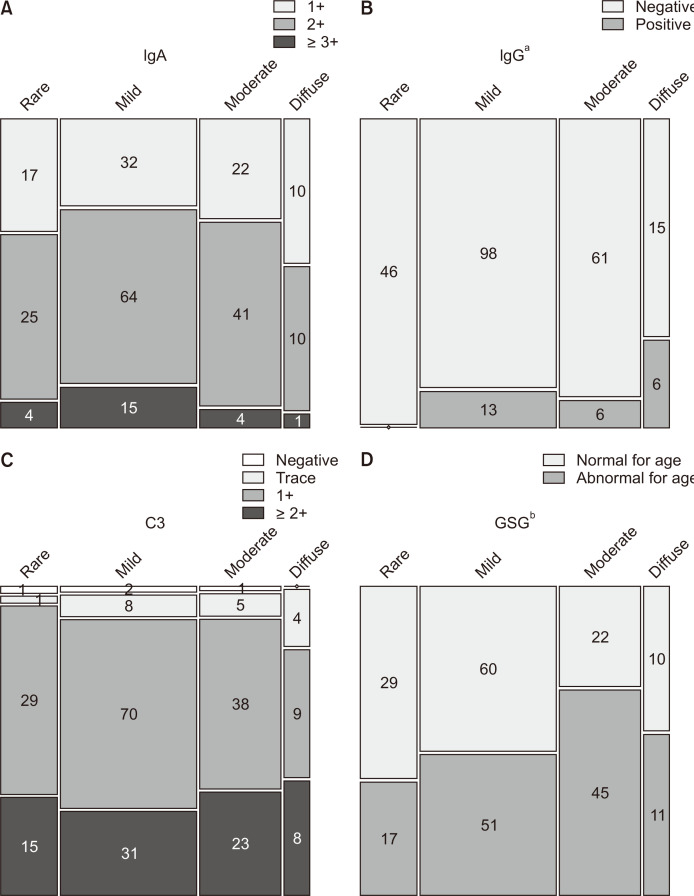
Figure 4
Degree of proteinuria based on the Oxford classification.

Figure 5
The association of endocapillary hypercellularity and crescents and their implications on proteinuria.
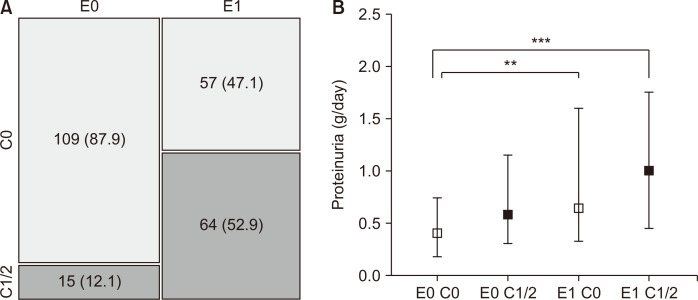
Table 1
| Characteristic | Rare FPE (n = 46) | Mild FPE (n = 111) | Moderate FPE (n = 67) | Diffuse FPE (n = 21) | P value |
|---|---|---|---|---|---|
| Age (yr) | 33 (23-49) | 38 (30-48) | 40 (31-46) | 39 (31-49) | 0.655 |
| Male | 30 (65.2) | 64 (57.7) | 36 (53.7) | 13 (61.9) | 0.656 |
| HTN | 14 (30.4) | 33 (29.7) | 22 (32.8) | 8 (38.1) | 0.664 |
| Current smoker | 9 (19.6) | 20 (18.0) | 9 (13.4) | 3 (14.3) | 0.799 |
| BMI (kg/m2) | 24.0 (21.2-26.7) | 23.8 (21.0-26.4) | 23.2 (21.5-26.4) | 23.1 (21.4-26.5) | 0.870 |
| MAP (mmHg) | 89 (76-97) | 93 (83-97) | 93 (83-97) | 93 (82-107) | 0.403 |
| WBC count (/μL) | 7,105 (5,933-8,165) | 6,710 (5,820-8,120) | 7,080 (5,590-8,720) | 6,640 (5,630-8,405) | 0.823 |
| Hemoglobin (g/dL) | 13.8 ± 1.6 | 13.7 ± 1.6 | 13.5 ± 1.8 | 12.8 ± 2.6 | 0.118a |
| Platelet count (/μL) | 264 (233-289) | 251 (213-291) | 245 (217-284) | 224 (200-262) | 0.122 |
| Protein (g/dL) | 6.9 (6.5-7.2) | 6.8 (6.3-7.2) | 6.7 (6.2-7.2) | 6.1 (4.8-6.9) | 0.002b |
| Albumin (g/dL) | 4.2 (4.0-4.4) | 4.2 (3.9-4.4) | 4.1 (3.8-4.3) | 3.7 (2.5-4.3) | 0.005b |
| BUN (mg/dL) | 13.8 (11.1-16.1) | 14.5 (12.6-17.8) | 15.7 (12.2-19.3) | 16.7 (12.3-27.5) | 0.064b |
| Creatinine (mg/dL) | 0.84 (0.70-0.99) | 0.87 (0.70-1.10) | 1.00 (0.80-1.20) | 1.20 (0.85-1.70) | < 0.001b |
| eGFR (mL/min per 1.73 m2) | 103.5 (93.2-123.4) | 108.0 (77.8-122.3) | 88.4 (64.5-112.6) | 71.1 (37.2-107.9) | < 0.001b |
| Uric acid (mg/dL) | 5.8 (4.4-6.5) | 5.7 (4.8-6.6) | 6.2 (4.6-7.4) | 7.0 (5.7-9.1) | 0.008b |
| Calcium (mg/dL) | 9.3 (9.2-9.6) | 9.2 (8.8-9.4) | 9.1 (8.7-9.5) | 8.6 (7.9-9.1) | < 0.001 |
| Corrected calcium (mg/dL) | 9.1 ± 0.3 | 9.0 ± 0.4 | 9.0 ± 0.5 | 8.9 ± 0.5 | 0.256 |
| Phosphorus (mg/dL) | 3.5 ± 0.5 | 3.6 ± 0.6 | 3.5 ± 0.5 | 3.8 ± 0.8 | 0.218 |
| Urine protein (g/day) | 0.38 (0.16-0.70) | 0.60 (0.35-1.25) | 0.60 (0.33-1.45) | 0.74 (0.38-2.70) | 0.003b |
| PCR (g/g) | 0.30 (0.15-0.52) | 0.55 (0.28-1.13) | 0.58 (0.34-1.12) | 1.22 (0.29-2.21) | < 0.001b |
| Total glomeruli (counts) | 32 (18-42) | 23 (16-35) | 22 (13-34) | 22 (14-26) | 0.038b |
| Glomerulosclerosis (%) | 5.9 (0.0-16.3) | 8.7 (1.3-23.1) | 18.8 (6.7-42.1) | 16.2 (5.2-33.7) | < 0.001b |
| GSG abnormal for age | 17 (37.0) | 51 (45.9) | 45 (67.2) | 11 (52.4) | 0.008b |
| Oxford classification | |||||
| M1 | 14 (30.4) | 49 (44.1) | 44 (65.7) | 11 (52.4) | 0.002b |
| E1 | 23 (50.0) | 52 (46.8) | 35 (52.2) | 11 (52.4) | 0.899 |
| S1 | 34 (73.9) | 94 (84.7) | 57 (85.1) | 16 (76.2) | 0.321 |
| T1 | 4 (8.7) | 25 (22.5) | 19 (28.4) | 6 (28.6) | 0.009b,c |
| T2 | 0 (0.0) | 2 (1.8) | 6 (9.0) | 0 (0.0) | |
| C1 | 13 (28.3) | 37 (33.3) | 23 (34.3) | 3 (14.3) | 0.280c |
| C2 | 1 (2.2) | 1 (0.9) | 0 (0.0) | 1 (4.8) |
Data are presented as mean ± standard deviation, median (interquartile range), or number (%) as appropriate.
BMI, body mass index; BUN, blood urea nitrogen; C, crescents; E, endocapillary hypercellularity; eGFR, estimated glomerular filtration rate; FPE, foot process effacement; GSG, globally sclerotic glomeruli; HTN, hypertension; M, mesangial hypercellularity; MAP, mean arterial pressure; PCR, urine protein to creatinine ratio; S, segmental glomerulosclerosis; T, tubular atrophy/interstitial fibrosis; WBC, white blood cell.
aP value was calculated using one-way ANOVA. In other cases, it was calculated using the Kruskal–Wallis test. bStatistical significance in the trend test. For continuous variables, the Jonckheere–Terpstra test was used. For categorical variables, Cochran–Armitage (only for 2 × k tables) or linear-by-linear test was used. cFisher’s exact test was used.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















