| Kidney Res Clin Pract > Epub ahead of print |
Abstract
Background
Methods
Results
Notes
Funding
This work was supported by the Health Commission of Minhang District, Shanghai (No. 2021MW04), the Minhang District medical characteristic specialty construction project (No. 2020MWTZA01), and the Cooperation Program of Fudan University-Minhang District Joint Health Center (No. 2021FM21).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: YG, CY, JN
Data curation: WF, XZ, XC
Formal analysis: WF, XZ, QW
Investigation: WF, Lihong Zhang, ZY, XC, KZ, WD, HQ, JZ, Liming Zhang, SZ
Methodology: WF, XZ, JN
Project administration: QG, CY, HQ, SZ, JN
Supervision: QG, CY, WD, JZ, Liming Zhang, JN
Validation: CY
Writing – original draft: WF, XZ
Writing – review & editing: WF, XZ, JN
All authors read and approved the final manuscript.
Figure 2.
Correlations between pure-tone averages (PTA) and each test.
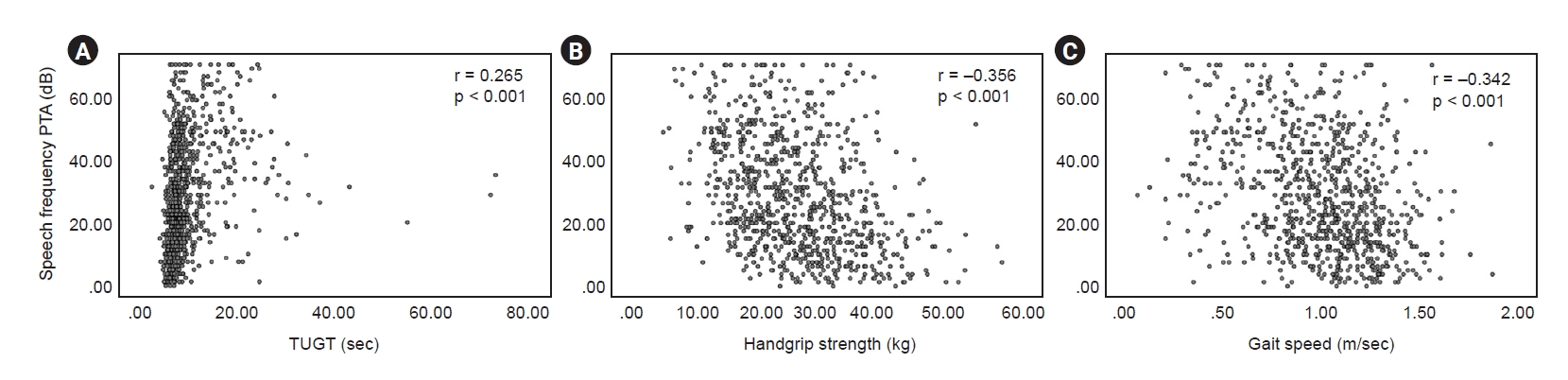
Figure 3.
The prevalence of hearing loss depends on physical performance by age.
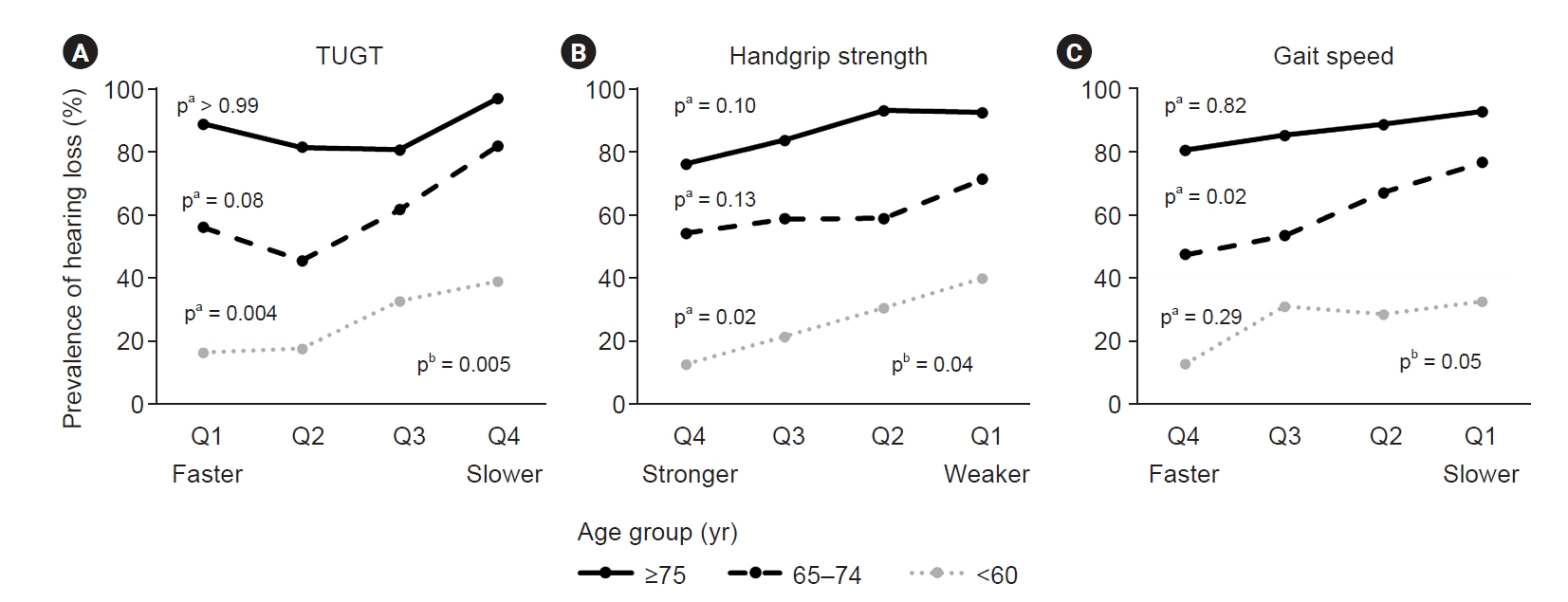
Table 1.
Table 2.
| Physical performance | Q1 | Q2 | Q3 | Q4 | p for trend |
|---|---|---|---|---|---|
| TUGT(s) | |||||
| Number | 216 (25.8) | 204 (24.3) | 209 (24.9) | 209 (24.9) | |
| Crudea | 1.00 | 1.82 (1.21–2.74) | 3.44 (2.29–5.15) | 8.49 (5.48–13.14) | 0.001 |
| Model 1b | 1.00 | 1.24 (0.80–1.93) | 1.78 (1.14–2.80) | 2.97 (1.81–4.88) | 0.004 |
| Model 2c | 1.00 | 1.15 (0.73–1.81) | 1.69 (1.07–2.70) | 2.87 (1.69–4.88) | 0.005 |
| Handgrip strength (kg) | |||||
| Number | 209 (24.9) | 211 (25.2) | 209 (24.9) | 209 (24.9) | |
| Crudea | 1.00 | 0.53 (0.35–0.79) | 0.36 (0.24–0.54) | 0.18 (0.12–0.28) | 0.048 |
| Model 1b | 1.00 | 0.62 (0.39–0.97) | 0.49 (0.30–0.81) | 0.33 (0.19–0.58) | 0.04 |
| Model 2c | 1.00 | 0.61 (0.39–0.97) | 0.46 (0.28–0.76) | 0.31 (0.17–0.56) | 0.04 |
| Gait speed (m/sec) | |||||
| Number | 209 (24.9) | 209 (24.9) | 211 (25.2) | 209 (24.9) | |
| Crudea | 1.00 | 0.38 (0.25–0.57) | 0.25 (0.17–0.38) | 0.16 (0.11–0.25) | 0.06 |
| Model 1b | 1.00 | 0.55 (0.35–0.86) | 0.51 (0.32–0.80) | 0.36 (0.22–0.58) | 0.04 |
| Model 2c | 1.00 | 0.53 (0.33–0.84) | 0.52 (0.32–0.83) | 0.35 (0.21–0.58) | 0.05 |
Table 3.
| Physical performance | Q1 | Q2 | Q3 | Q4 | p for trend |
|---|---|---|---|---|---|
| TUGT(s) | |||||
| Number | 83 (25.2) | 83 (25.2) | 82 (24.9) | 82 (24.9) | |
| Crudea | 1.00 | 1.087 (0.49–2.42) | 2.419 (1.16–5.05) | 3.154 (1.53–6.52) | 0.04 |
| Model 1b | 1.00 | 1.088 (0.48–2.48) | 1.975 (0.92–4.23) | 2.459 (1.16–5.21) | 0.04 |
| Model 2c | 1.00 | 1.063 (0.46–2.48) | 1.900 (0.86–4.19) | 2.398 (1.04–5.56) | 0.004 |
| Handgrip strength (kg) | |||||
| Number | 82 (24.9) | 84 (25.5) | 82 (24.9) | 82 (24.9) | |
| Crudea | 1.00 | 0.666 (0.35–1.26) | 0.418 (0.21–0.83) | 0.230 (0.11–0.50) | 0.01 |
| Model 1b | 1.00 | 0.705 (0.35–1.41) | 0.408 (0.18–0.93) | 0.230 (0.09–0.58) | 0.01 |
| Model 2c | 1.00 | 0.704 (0.35–1.44) | 0.353 (0.15–0.83) | 0.224 (0.08–0.59) | 0.02 |
| Gait speed (m/sec) | |||||
| Number | 82 (24.9) | 83 (25.2) | 83 (25.2) | 82 (24.9) | |
| Crudea | 1.00 | 0.829 (0.43–1.61) | 0.929 (0.48–1.79) | 0.316 (0.14–0.69) | 0.20 |
| Model 1b | 1.00 | 0.919 (0.46–1.83) | 0.962 (0.49–1.89) | 0.349 (0.16–1.78) | 0.23 |
| Model 2c | 1.00 | 1.004 (0.49–2.07) | 1.029 (0.51–2.09) | 0.336 (0.14–0.79) | 0.29 |
Table 4.
| Physical performance | Q1 | Q2 | Q3 | Q4 | p for trend |
|---|---|---|---|---|---|
| TUGT(s) | |||||
| Number | 104 (25.6) | 101 (24.8) | 101 (24.8) | 101 (24.8) | |
| Crudea | 1.00 | 0.663 (0.38–1.15) | 1.261 (0.72–2.20) | 3.423 (1.82–6.44) | 0.037 |
| Model 1b | 1.00 | 0.637 (0.36–1.12) | 1.006 (0.56–1.80) | 2.726 (1.42–5.22) | 0.061 |
| Model 2c | 1.00 | 0.542 (0.30–0.98) | 0.929 (0.51–1.70) | 2.464 (1.23–4.95) | 0.082 |
| Handgrip strength (kg) | |||||
| Number | 104 (25.6) | 100 (24.6) | 102 (25.1) | 101 (24.8) | |
| Crudea | 1.00 | 0.583 (0.33–1.04) | 0.579 (0.33–1.03) | 0.485 (0.27–0.86) | 0.127 |
| Model 1b | 1.00 | 0.610 (0.33–1.12) | 0.632 (0.32–1.25) | 0.523 (0.25–1.08) | 0.133 |
| Model 2c | 1.00 | 0.569 (0.30–1.06) | 0.586 (0.29–1.19) | 0.464 (0.22–0.99) | 0.126 |
| Gait speed (m/sec) | |||||
| Number | 101 (24.8) | 102 (25.1) | 103 (25.3) | 101 (24.8) | |
| Crudea | 1.00 | 0.623 (0.34–1.15) | 0.357 (0.20–0.65) | 0.282 (0.16–0.52) | 0.028 |
| Model 1b | 1.00 | 0.693 (0.37–1.30) | 0.432 (0.23–0.80) | 0.332 (0.18–0.62) | 0.017 |
| Model 2c | 1.00 | 0.647 (0.34–1.24) | 0.450 (0.24–0.85) | 0.338 (0.18–0.66) | 0.018 |
Table 5.
| Physical performance | Q1 | Q2 | Q3 | Q4 | p for trend |
|---|---|---|---|---|---|
| TUGT(s) | |||||
| Number | 25 (24.8) | 26 (25.8) | 25 (24.8) | 25 (24.8) | |
| Crudea | 1.00 | 0.573 (0.12–2.70) | 0.545 (0.12–2.58) | 3.273 (0.32–33.84) | 0.184 |
| Model 1b | 1.00 | 0.308(0.05–1.77) | 0.210 (0.04–1.22) | 0.605 (0.05–7.99) | 0.748 |
| Model 2c | 1.00 | 0.299(0.04–2.19) | 0.190 (0.02–1.71) | 0.806 (0.04–15.73) | >0.99 |
| Handgrip strength (kg) | |||||
| Number | 25 (24.8) | 27 (26.7) | 24 (23.8) | 25 (24.8) | |
| Crudea | 1.00 | 1.087 (0.14–8.36) | 0.435 (0.07–2.63) | 0.275 (0.05–1.53) | 0.098 |
| Model 1b | 1.00 | 0.760 (0.09–6.74) | 0.283 (0.01–7.16) | 0.248 (0.01–5.66) | 0.07 |
| Model 2c | 1.00 | 0.991 (0.08–11.89) | 0.248 (0.01–12.67) | 0.192 (0.01–9.63) | 0.10 |
| Gait speed (m/sec) | |||||
| Number | 25 (24.8) | 25 (24.8) | 26 (25.8) | 25 (24.8) | |
| Crudea | 1.00 | 0.638 (0.10–4.19) | 0.478 (0.08–2.88) | 0.348 (0.06–1.99) | 0.03 |
| Model 1b | 1.00 | 0.345 (0.03–3.49) | 1.774 (0.22–14.62) | 1.076 (0.13–9.01) | 0.66 |
| Model 2c | 1.00 | 0.177 (0.01–2.32) | 1.704 (0.15–19.39) | 0.823 (0.07–9.90) | 0.82 |
References
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 512 View
- 20 Download
- ORCID iDs
-
Weifeng Fan

https://orcid.org/0000-0002-5931-7630Xiaojing Zhong

https://orcid.org/0000-0001-8810-9411Qing Wu

https://orcid.org/0000-0001-9955-9629Lihong Zhang

https://orcid.org/0000-0002-4759-8404Zhenhao Yang

https://orcid.org/0000-0001-6195-1182Yong Gu

https://orcid.org/0000-0002-2861-6937Qi Guo

https://orcid.org/0000-0003-3795-1988Xiaoyu Chen

https://orcid.org/0000-0002-2521-799XChen Yu

https://orcid.org/0000-0002-7779-6188Kun Zhang

https://orcid.org/0000-0002-0527-0873Wei Ding

https://orcid.org/0000-0003-0482-5977Hualin Qi

https://orcid.org/0000-0003-4716-0901Junli Zhao

https://orcid.org/0000-0002-7780-0511Liming Zhang

https://orcid.org/0000-0002-0414-0292Suhua Zhang

https://orcid.org/0000-0001-6776-3617Jianying Niu

https://orcid.org/0000-0002-3323-5071 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















